The compounds containing the multiple bonds are named according to the following rules.
1. The longest continuous chain containing the carbon atoms involved in the multiple bonds is selected.
2. While writing the name of the alkene or alkyne, the suffix ‘ane’ of the corresponding alkane is replaced by ‘ene’ or ‘yne’ respectively.
3. If the multiple bond occurs twice in the parent chain, the alkene and alkyne are called diene and diynerespectively.
4. The numbering of atoms in parent chain is done in such a way that the carbon atom containing the double or triple bond gets the lowest number.
Correct
Wrong
5. All the rules for naming the side chains or substituents are similar to alkanes.
Important Note:
If both double and triple bonds are present in a parent chain, the following rules should be remembered.
1. The terminal ‘e’ in the name is dropped when it is followed by the suffix beginning with ‘a’, ‘i’, ‘o’, ‘u’ or ‘y’
2. Numbers as low as possible are given to double bond and triple bond as a set, even though this may at times give ––yne a lower number than –ene.
However, if a choice is there, preference for lower locants is given to double bond(–ene).
The name cannot be Pent – 2 – en – 4 – yne because lowest set is 1,3 rather than 2,4.
Here are some more examples:
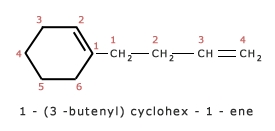
3. In case of cyclic alkenes, the position of double bond is always given the number 1.
The name of the compound cannot be 2,3 – Dimethyl cyclohex – 1 – ene because of lowest set rule.
compare the set (1,6) with (2,3), the former is correct because 1 is lower than 2.